Eye drop recall
However one reported buying EzriCare at a Costco warehouse. Web CNN Global Pharma Healthcare is issuing a recall of its Artificial Tears Lubricant Eye Drops that were distributed by EzriCare and Delsam Pharma due to.

27jcbcjyde0vam
If you have questions about the products being recalled you can contact EzriCares distributor at 518-738-7602 or.

. The drops have not been linked to illness the. A majority of those affected reported using preservative-free EzriCare. Web An alarming outbreak of extensively drug-resistant bacteria linked to eye drops has now sickened 68 people across 16 states according to the latest update from.
Health officials are alerting consumers about two more recalls of eyedrops due to contamination risks that could lead to vision problems and. Five lots of Clear Eyes eye drops have been recalled by Teva Pharmaceuticals the latest in a run of recalls of prescription and over. Web WASHINGTON US.
Web The eye drops were recalled in early February after the Centers for Disease Control and Prevention linked them to an outbreak of Pseudomonas aeruginosa. Web On Thursday the maker of the eyedrops recalled them because of possible contamination. Web 4 hours agoPharmedica USA in March recalled Purely Soothing 15 MSM Drops out of concern that the product is not sterile.
Web Pharmedica USA issued a voluntary worldwide recall of two lots of its Purely Soothing 15 MSM eye drops the Food and Drug Administration announced March 3. Web A rare strain of bacteria found in recalled eyedrops has been linked to dozens of infections as well as cases of vision loss surgical removal of eyeballs and one. Web 22 hours agoEzriCare eye drops had been recalled back in February after being linked to 55 bacterial cases in 12 states.
Web At the time of the recall there were 55 reports of adverse reactions to the drops including eye infections permanent vision loss and one death from a. The manufacturer Global Pharma. Thirty-five patients were connected to four healthcare.
Officials are reporting two more deaths and additional cases of vision loss linked to eyedrops tainted with a drug-resistant bacteria. Web March 21 2023 349 PM. The company recalled six lots.
Web The companies involved in the recalls are Phoenix-based Pharmedica and Florida-based Apotex. Web On March 1 Apotex recalled prescription eye drops used to reduce eye pressure in people with glaucoma or ocular hypertension. Web Most cases linked to the outbreak involved eye drops purchased online before the recall.
Pharmedica is recalling its Purely Soothing 15 MSM Drops. Web The FDA and CDC are advising patients to stop using EzriCare Artificial Tears an over-the-counter brand of eye drops. Web 18 hours agoUS.
Web The Food and Drug Administration posted separate recall notices for certain eyedrops distributed by Pharmedica and Apotex after the companies said they are. Web Global Pharma Healthcare is voluntarily recalling all lots within expiry of their Artificial Tears Lubricant Eye Drops distributed by EzriCare LLC- and Delsam Pharma to the. Web 23 hours agoHealth Manufacturer recalls eyedrops after possible link to bacterial infections Per the CDCs latest update 68 patients across 16 states have been infected.
Web The eye drops could be contaminated.

What Is Happening With The Eye Drop Recall

Contamination Risks That Could Cause Injuries Cited In Recalls Of Two Eye Drop Brands Fox31 Denver

Ei8ybhiqm2qccm

Altaire Recalls Eye Drops Ointments Sold At Walgreens Healthcare Packaging
Eye Drop Recall Walmart Cvs Products May Not Be Sterile
02mf3hh9wahuam

Eye Drop Recall Everything You Need To Know Right Now

O N2vpc3liz1vm

Eye Drop Recall More Deaths Injuries Linked To Bacteria 9news Com

Fda Eye Drops Recall Lawyer Contaminated Artificial Tears Attorney Hastings Law Firm Medical Malpractice Lawyers
Pgn5ycba5ywmm

Rohto Eye Drops Recall Due To Sterility Concerns Aboutlawsuits Com

Eye Drops Being Recalled Swiftcurrentonline Com Local News Weather Sports Free Classifieds And Job Listings
Aezpbzfix Pwom
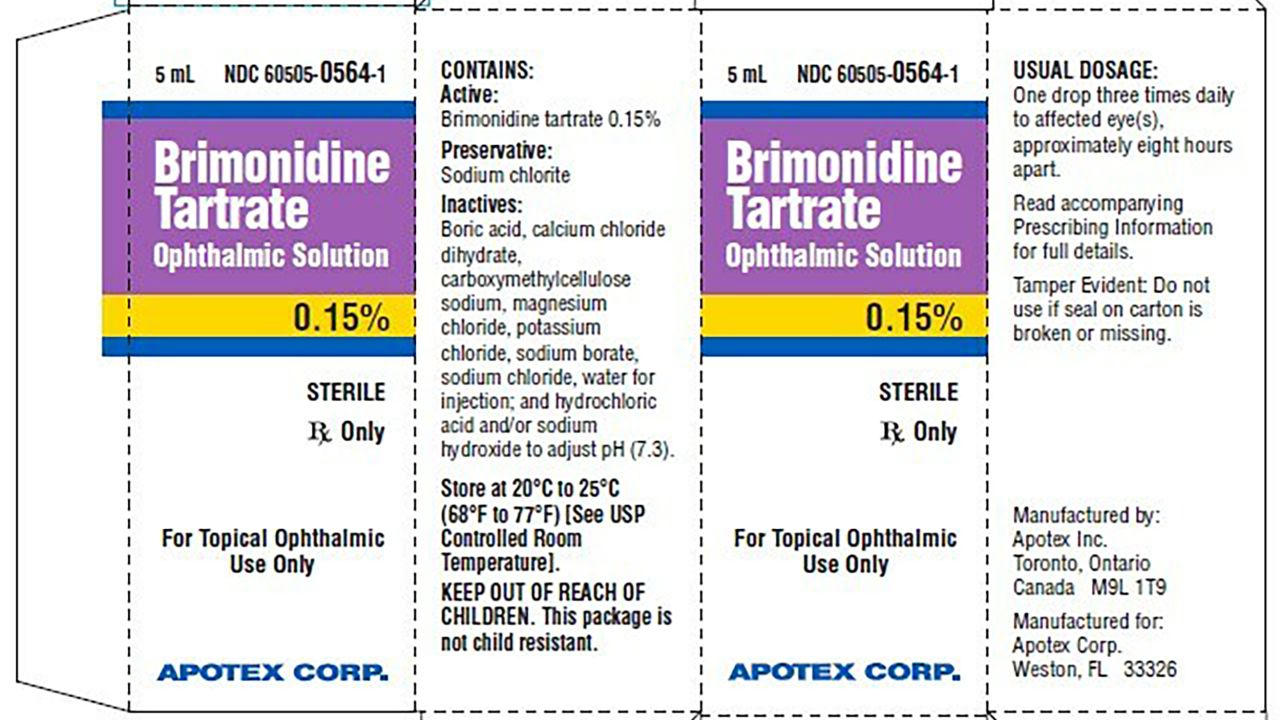
O N2vpc3liz1vm
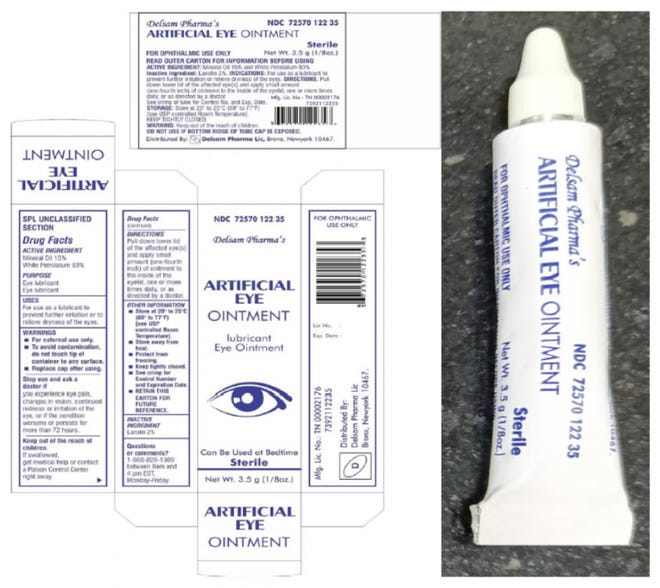
Delsam Pharma Eye Ointment Recalled Over Eye Infection Blindness Risk

Recall Cdc Alert Prompt Concern Over Preservative Free Eye Drops